Fouling of cooling water system
Scale, slime and corrosion (rust) which arise in the cooling water produce problems such as lowering the cooling efficiency, puncture accidents in the refrigerator, blockage of the piping, and breeding of algae and bacteria. The photographs on the right (【Fig-01】, 【Fig-02】) show typical fouling of the cooling water system.
Such fouling progresses inside the waterway. It causes increases in heat transfer resistance, water flow reduction due to the blockage of the flow path and increases in friction resistance. This results in poor, non-uniform and unstable heat transfer from mold to the cooling water.
Corrosion (rust)
The rust is generated by the corrosion of the metal. When the metal is soaked in the water, minute local anodes and local cathodes are formed on the surface of the metal due to impurities in the metal, non-uniform concentration of dissolved oxygen or uneven temperatures. On the anode part, the iron becomes a ferrous ion of the divalence and dissolves into water. And on the cathode part, hydroxide ion (OH-) is formed by electrons discharged during the anodic reaction, with oxygen and water.As a whole, iron corrodes by the reaction of Fe+H2O+1/2O2 →Fe(OH)2.
The iron hydroxide (Fe(OH)2) is further oxidized to become red rust (Fe2O3 ∙nH2O), black rust (Fe3O4∙nH2O), and blue rust (FeO∙nH2O) and accumulates on the metal surface (【Fig-03】).
Corrosion promoting factors
The dissolved oxygen is indispensable for the corrosion to progress. The oxygen is easily dissolved into the water when water comes into contact with air.
In cooling towers, the oxygen dissolves into water from air rapidly, because the contact surface area of water and air is large in order to promote the evaporation. Corrosion easily progresses in cooling-tower water systems since they contain sufficiently dissolved oxygen. Therefore corrosion occurs frequently in pipes and devices in which cooling-tower water is supplied.
Dissolved oxygen is the major cause of the corrosion in the cooling water system. Corrosion does not progress unless oxygen reaches metal surfaces. 【Fig-04】 (next page) shows the relationship between the concentration of dissolved oxygen and the corrosion rate. From this figure, you can see that the corrosion rate is in proportion to the dissolved oxygen concentration.

【Fig-01 Cross section of PVC pipe】
Used in a opening cooling tower water circuit for 5 years.
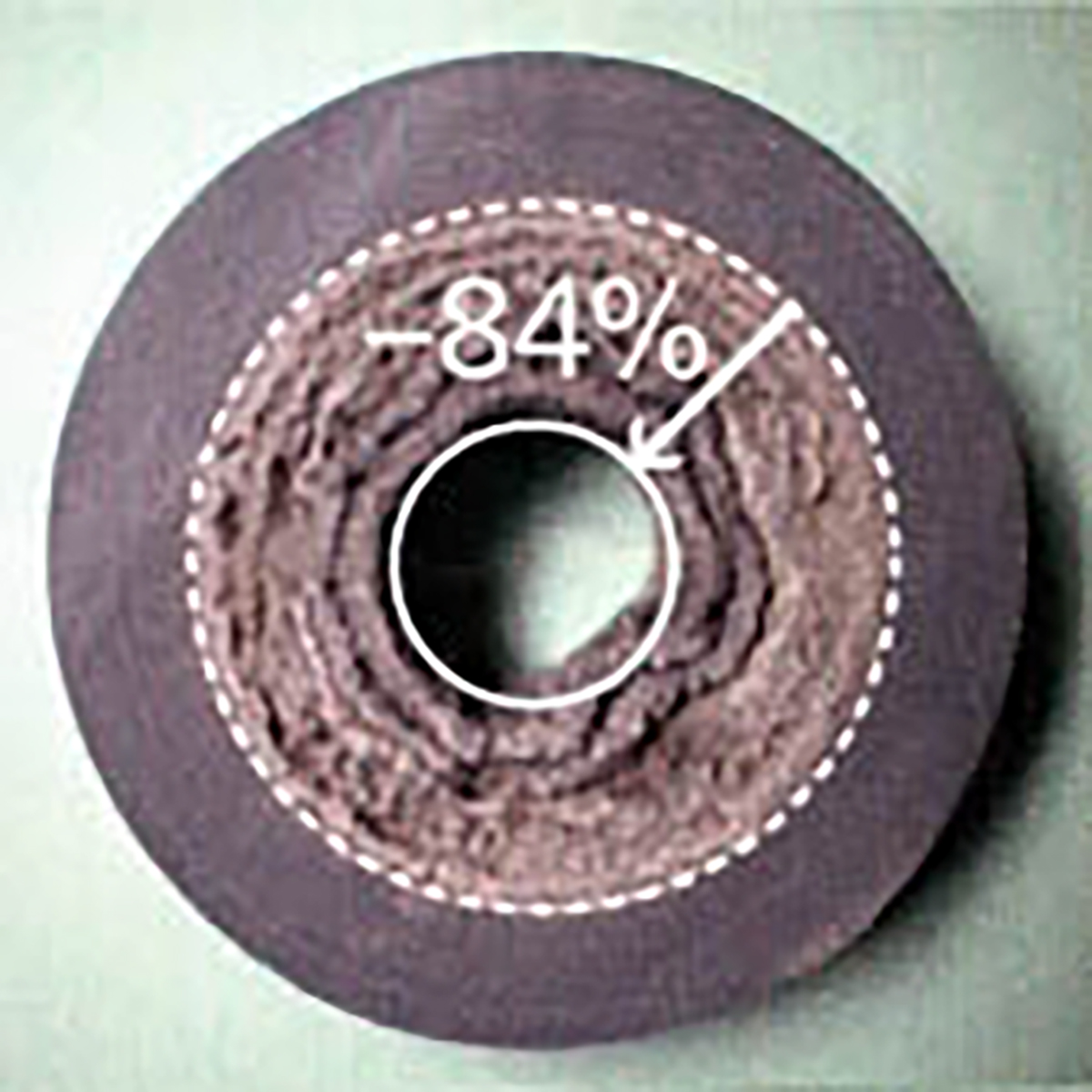
【Fig-02 Cross section of brass elbo】
Used in a closing cooling tower water circuit for 6 months at 130--135℃.
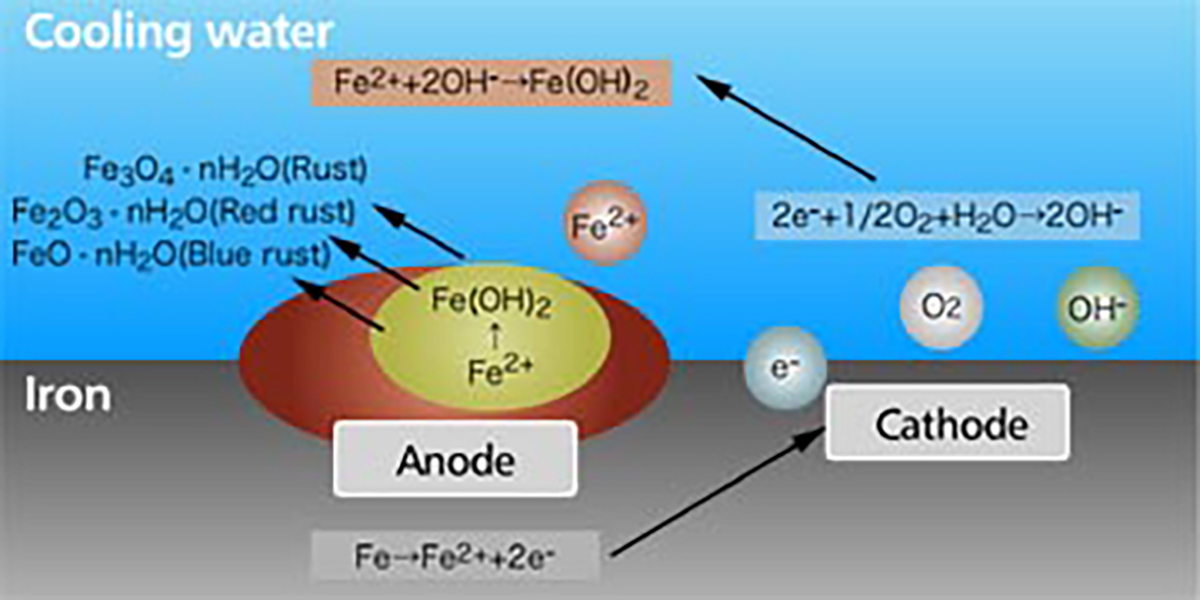
【Fig-03 Corrosion process of iron in water】
Scale
Scale is a stone-like solid formed by the calcification of calcium carbonate, magnesium carbonate or silica which dissolves in water. Through the evaporation of cooling-tower water, the concentration of these dissolved carbonates increases, as does pH. This causes the scale formation because the solubility of these materials decreases with increasing temperature and/or pH.
The solubility of the carbonate decreases when pH rises through the condensation of cooling water, because the lower the temperature and pH are, the larger the solubility of carbonates becomes. Moreover, the temperature of the cooling water for injection molding is relatively high.
Condensation in the cooling tower is the only factor that sometimes makes the concentration of dissolved saline exceeds the saturation levels. Usually in addition to the concentration, the temperature and pH also rise reducing the solubility, and the cooling water easily becomes super saturated. Thus, saline crystallizes and adheres to the walls as scale.
Examples of this process are shown in 【Fig-05】. In a typical case, cooling water with an initial pH of 7.5, temperature of 25℃ and calcium hardness of 50 ppm was chosen. When its concentration increased five times, the water became super saturated even if the temperature did not rise. When the concentration increased no more than double, the water went into super saturation if its temperature rose to 45℃.
Slime
Slime is a generic name given to material made by micro-organisms in water, such as algae, bacteria and fungus. Since cooling water contains enough nutrition and its temperature is favorable for such micro-organisms to grow, they prosper in the cooling water system and adhere to the surface of pipes or heat exchangers. The effect of slime on the cooling system is similar to corrosion and scale.
In addition to this, slime sometimes forms a hot-bed of disease germs, such as Legionella which is known to cause the Legionnaire’s disease.
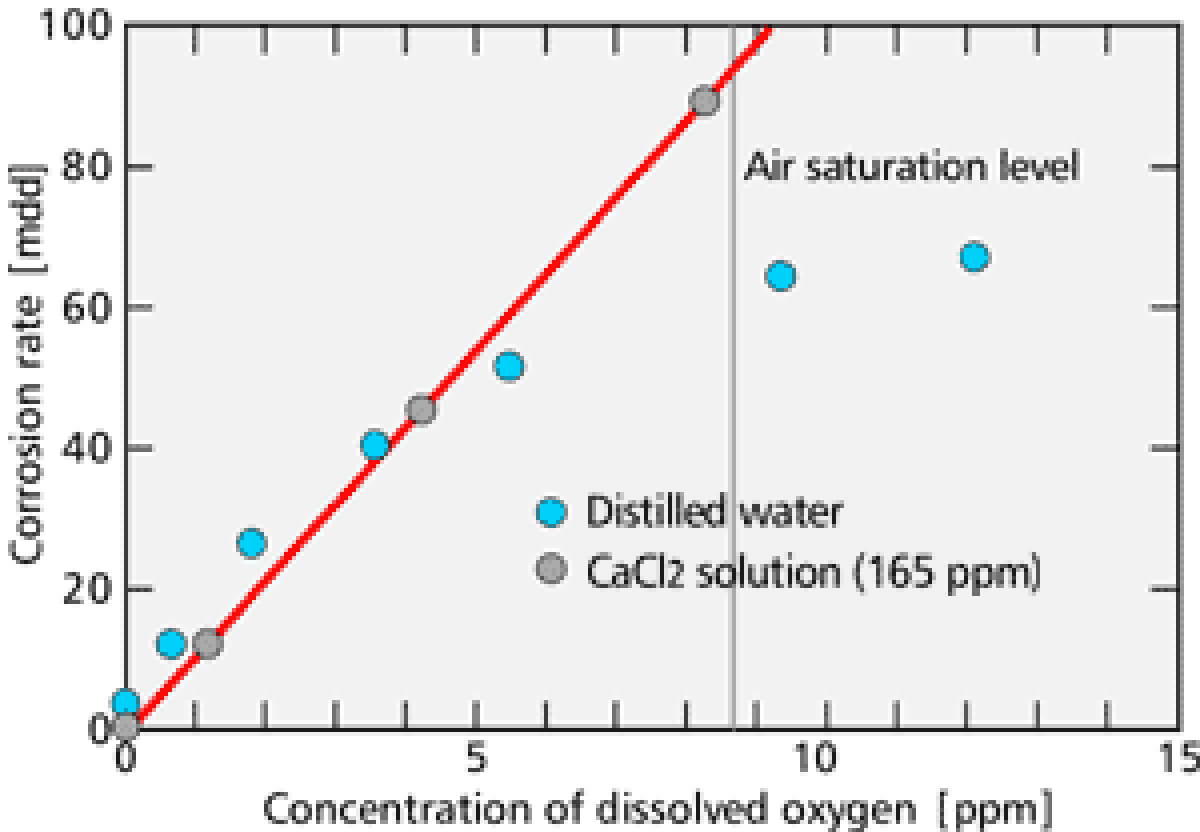
【Fig-04 Relation between oxygen concentration
and corrosion rate (25℃)】
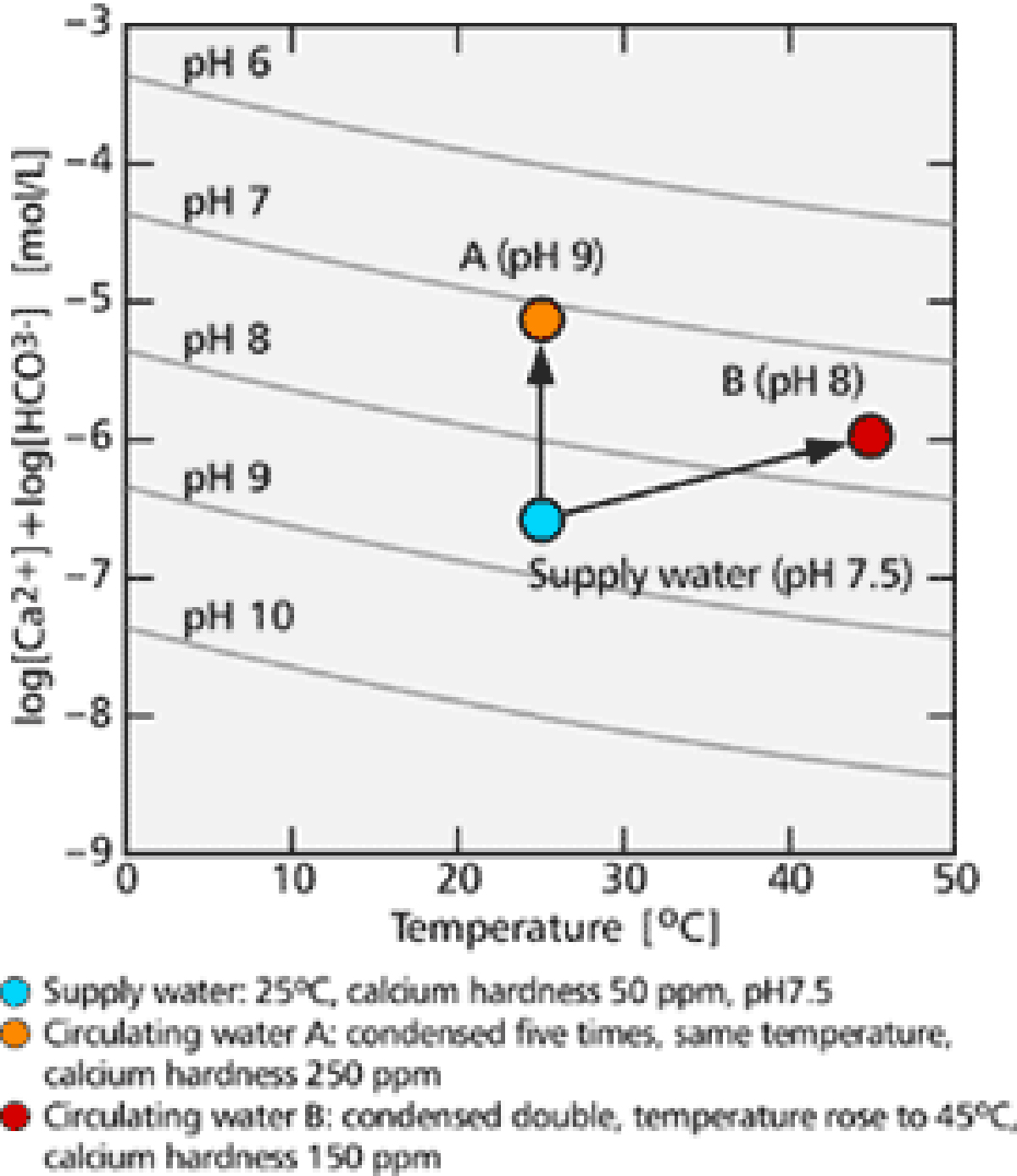
【Fig-05 Relation between scale precipitation and temperature, pH】
